Back Titration Method
In such situations we can often use a technique called back titration. A tricky but common twist to the typical titration question.
Difference Between Back Titration And Direct Titration Definition Examples Applications
Top things to consider.

. Aspirin- chemically known as acetyl salicylic acid is a weak. Back Titration Technique This reaction cannot be used directly to titrate the CaCO 3 because it is very slow when the reaction is close to completion endpoint. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent whereas a direct titration.
There are certain principles that need to be followed to perform back titration. In the back-titration method a measured amount of an excess standard EDTA solution is added to the sample. This video will give you the basics of the back titration.
- one of the reactants is volatile for example ammonia. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Procedure Weigh out about 25 g of the unknown carbonate Dissolve the unknown carbonate in 50 cm 3 of 2M HCl an excess of acid - it will react with all of the carbonate and there will be.
In back titration we use two reagents - one that reacts with the original sample lets call it A and second lets call it B. You will use the NaOH you standardized last week to. 11 The iodometric back titration method is applicable to all types of waters but is primarily used for wastewater because it eliminates any contact between the full concentration of liberated.
- an acid or a base is an insoluble salt for example calcium carbonate - a particular reaction is too slow -. Add the excess amount of reagent to an analyte. Back titration is an analytical chemistry technique which allows the user to find the concentration of a reactant of unknown concentration by reacting it with an excess volume of.
The back titration in. Back titrations are used when. A student was asked to determine the mass in grams of calcium carbonate present in a 0125 g sample of.
Make chemistry easy today with 1 chemistry help. Wash the egg shell. The analyte ion combines with EDTA.
An automatic back titration method for microchemical analysis is introduced which is based on conventional volumetric analysiss principle and the use of flow injection analysis apparatus for. Back Indirect Titration to Determine the Amount of an Insoluble Salt. In this video were going to learn what is back titration and how to solve back titration calculation questions with the model method.
Determination of Aspirin using Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration. Ad Dont fall behind in chemistry. Back titration was the method used to determine the concentration of aspirin by reacting it with known amount of excess base.
After the reaction is complete the excess.
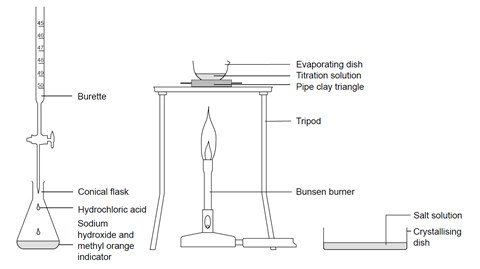
Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education

What Is The Use Of Titration How Do You Explain Back Titration Quora

0 Response to "Back Titration Method"
Post a Comment